
19–22 Although these observations troubled most investigators, the value for p K 1′ in plasma is routinely adjusted through the use of nomograms, tables, or polynomial equations to account for changes in plasma pH, protein concentration, and sodium concentration. A number of studies have demonstrated that the value for p K 1′ in plasma is influenced by pH, protein concentration, and sodium concentration. 6 This finding shows that the Henderson-Hasselbalch equation cannot be accurately applied to mammalian blood cooled in vitro or to poikilothermic animals.ĭetermination of accurate p K 1′ values for plasma has been more problematic, because the experimental value for p K 1′ (the apparent dissociation constant) in plasma differs marginally from the value obtained in aqueous nonplasma solutions. It was unexpectedly found, however, that the measured ΔpH/ΔT of plasma was −0.015 to −0.020 units/° C, a value three to four times the −0.005 units/° C predicted by p K 1′. It had been expected that p K 1′, like all equilibrium constants based on molalities, would be altered by changes in ionic strength and temperature. 16 In 1925, p K 1′ (and therefore pH) was shown to be influenced by ionic strength (μ) 17 and temperature, 18 the Δp K 1′/ΔT being −0.005 units/° C over a temperature range of 20° to 38° C.
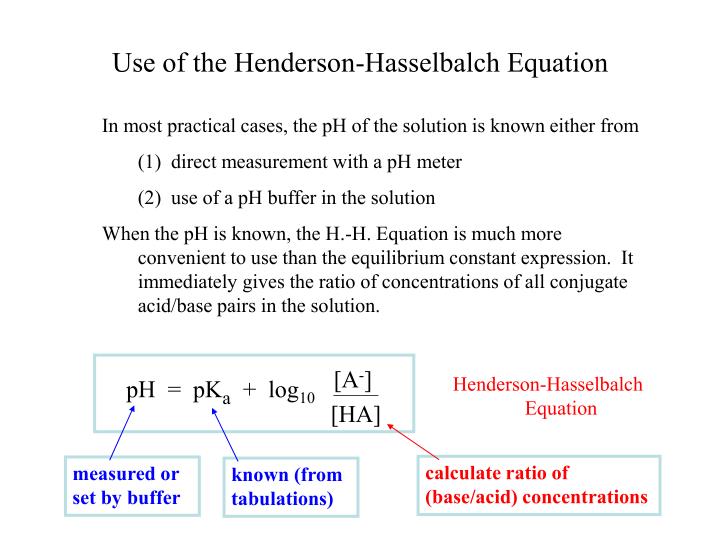
It was evident as early as 1922 that factors other than P co 2,, p K 1′, and S influence plasma pH. Metabolic alkalosis (increased extracellular base excess, actual HCO 3 − concentration, or standard HCO 3 −) Metabolic acidosis (decreased extracellular base excess, actual HCO 3 − concentration, or standard HCO 3 − ) Respiratory alkalosis (decreased P co 2) With the traditional Henderson-Hasselbalch approach, the following primary acid-base disturbances have been defined ( Figs. The Henderson-Hasselbalch equation has proved invaluable in aiding the understanding of mammalian acid-base physiology and is routinely used in the treatment of acid-base abnormalities.


 0 kommentar(er)
0 kommentar(er)
